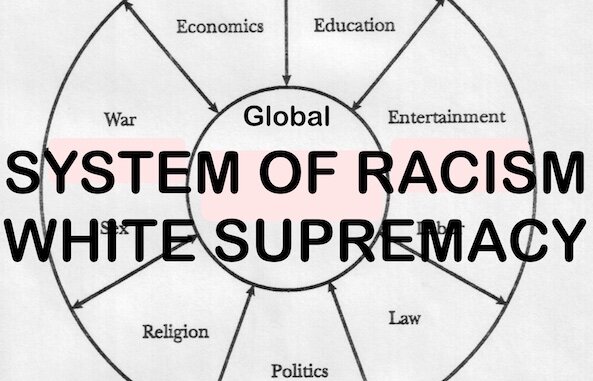
Report Shows Pfizer Ignored Deaths that Occurred During Its COVID Shot Clinical Trial, Failed to Fully Investigate in order to Provide a Misleading Picture of Safety
/From [HERE] and [MORE] Three participants in the Pfizer Covid vaccine trial died shortly after vaccination and their deaths were not fully investigated, it has been revealed.
The revelations come in a report from Pfizer released on July 1st by court order as part of the documents which the U.S. FDA relied on to grant emergency use authorisation for the Pfizer vaccine in December 2020. They add to worries that adverse effects of the vaccine in the clinical trials were not properly documented, giving a potentially misleading picture of the drug's safety.
One of the deceased participants, a 56-year-old woman known as subject #10071101, was given two doses of the vaccine on July 30th and August 20th 2020 and died from a cardiac arrest two months later. In Pfizer's report on the participant it says:
In the opinion of the investigator, there was no reasonable possibility that the cardiac arrest was related to the study intervention of clinical trial procedures, as the death occurred two months after receiving Dose 2. Pfizer concurred with the investigator's causality assessment.
However, it's not clear how the investigator and Pfizer can be so sure the death was unrelated to the vaccine when there was no autopsy and no thorough medical assessment. As Sonia Elijah, who has analysed the report and summarised parts of it for Trial Site News, comments:
The conclusion that "there was no reasonable possibility" the vaccine could have caused the fatal cardiac arrest because "death occurred two months after receiving Dose 2" is not only presumptuous but also lacks a robust medical assessment. This is evident by the further comment that "it was unknown if an autopsy was performed". Why was there no follow up or inquiry into whether an autopsy was performed?
A second deceased participant was a 60 year-old man known as subject #11621327, who died three days after his first dose of vaccine, given on September 10th 2020. The report says that the "probable cause of death was progression of atherosclerotic disease". However, the subject had no known history of the condition (though was reported as obese). While the investigator deemed there to be "no reasonable possibility" of a link with the vaccine, seeing that it happened three days after the injection and "autopsy results were not available at the time" of the report, again, it's hard to see what that conclusion is based on.
A third deceased participant was a 72 year-old man known as subject #11521497, who received the first vaccine dose on October 7th and 19 days later, on October 26th, was admitted to hospital because "he fainted in the middle of the night". Reported as a syncope (temporary loss of consciousness usually related to insufficient blood flow to the brain), 16 days later, on November 11th, he died. The investigator claimed there was "no reasonable possibility that the syncope was related to the study intervention" and Pfizer said the syncope was "most likely coincidental". But again, it's unclear what this assessment is based on as the cause of death was reported as "unknown" and neither the investigator nor Pfizer attempted to investigate.
There were also a number of serious but non-fatal adverse events among trial vaccine recipients, but oddly, in every case where the trial investigator deemed it to be possibly related to the vaccine, Pfizer "did not concur", according to Sonia Elijah.
For example, a 71-year-old woman known as subject #11421247 developed severe ventricular arrhythmias in the evening following her second dose on October 14th 2020. The trial investigator wrote that "there was reasonable possibility that the ventricular arrhythmia was related to the study intervention [vaccine]". However, Pfizer stated there was "not enough evidence to establish causal relationship". This was despite arrhythmia being one of the adverse events of special interest (AESI) listed by Pfizer in its analysis of post-authorisation adverse event reports. [MORE]