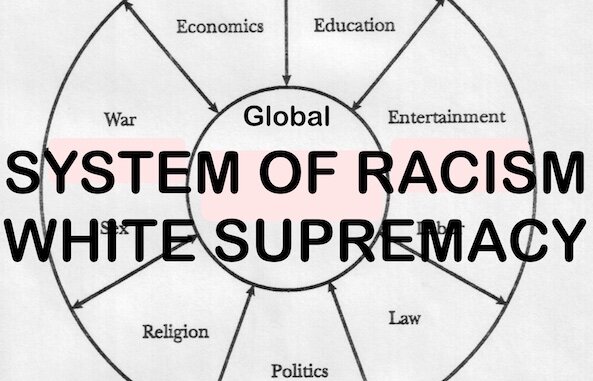
Contrary to Propaganda that "Vaccines" are Safe and Effective, Statistician Shows that Injections are More Dangerous than Driving a Car
/From [HERE] Every day we hear health authorities claiming that the current crop of novel platform COVID vaccines are “safe and effective.”
Last week we presented you with evidence that points to the increasing failure of the global mass vaccination program — at least in relation to its purported aim of stopping transmission and building herd immunity with a view to helping societies to exit the pandemic.
In the light of this evidence, there is no scientific support for the “effective” claim which should be deemed scientific (or medical) misinformation.
This week, we look closer at the other side of the coin, the claim widely made by authorities that these novel vaccines are “safe.”
Safety signals ignored in the early clinical trials
You’ll recall that regulatory agencies around the world, following suit behind the U.S. Food & Drug Administration, the UK’s Medicines and Healthcare Regulatory products Authority (MHRA) and the European Medicines Agency (EMA), issued ‘emergency use authorizations’ (EUAs) early on to BioNtech/Pfizer, Oxford/AstraZeneca, Moderna — and slightly later — to Janssen/Johnson & Johnson (J&J).
These were, by definition, experimental products being used on the public in a claimed “emergency” situation, one that the UK had already deemed was not the result of a “high consequence infectious disease.”
The data underpinning these decisions on both benefits and harms were very limited, derived from just two or three months’ worth of data. Efficacy data generally claiming in excess of 90% vaccine effectiveness was initially published in press releases by vaccine manufacturers.
Subsequently, safety and efficacy data for all three “vaccines” given EUAs in the U.S. were published in the prestigious New England Journal of Medicine: here for Pfizer, here for Moderna and here for J&J.
The stratospheric numbers reflecting efficacy — Pfizer and Moderna’s level pegging at 95% and J&J’s at 94.1%, like none that had ever been seen before for a vaccine — were enthusiastically delivered by the media to a public that had been conditioned to be fearful of the new coronavirus.
In countries that had control of the supply of experimental products, carefully crafted advertising generated very high levels of uptake for these experimental gene therapy products that were widely perceived as the surest way out of the surreal existence so many had endured for close to a year.
However, as we showed last week, these figures would not be sustained for long in the real world, outside the clinical conditions of trials, especially not in the face of immune escape and functional mutations (new variants).
J. Bart Classen M.D., an immunologist and vaccine adverse events researcher who previously worked at the National Institutes of Health and the National Institute for Allergy and infectious Diseases, headed by Dr. Anthony Fauci, has long been concerned about lack of transparencyaround vaccine data.
He argued in the BMJ as early as 1999, that the public should be “fully informed that vaccines … may have long term adverse effects,” that “proper safety studies were needed” and greater transparency might result in the “development of safer vaccine technology.”
Classen has recently turned his attention to the New England Journal of Medicine datasets of Pfizer, Moderna and J&J COVID “vaccines,” supported by some additional data issued by relevant FDA advisory committees.
I don’t bemoan Classen’s choice of journal to publish his results. The vast majority of high impact factor journals have done a great disservice to science by refusing to carry articles that are in any way critical of the novel “vaccines’.”
Classen’s analysis, accepted for publication in late August, can be found in the recently launched Trends in Internal Medicine, a minor journal that has yet to be listed in the U.S. National Library of Medicine catalog.
Classen — doing what he was trained to do while in service to Fauci’s department — did what any halfway decent researcher would initially do at the start of an investigation: compare severe adverse events in those who were injected with the ‘real thing’ from each of the 3 manufacturers as against those injected with saline placebos.
No further digging was required to spot a problem. The top line findings are summarized in Table 1 below.